Understanding how complexity arises from seemingly simple, ordered states is central to materials science—and few systems illustrate this better than undercooled metal alloys. In recent work, Dr. Martin Thuo and collaborators, including Andrew Martin, Ph.D., explored how deviation from the thermodynamic invariant eutectic point can drive spontaneous structural evolution in liquid metal systems.
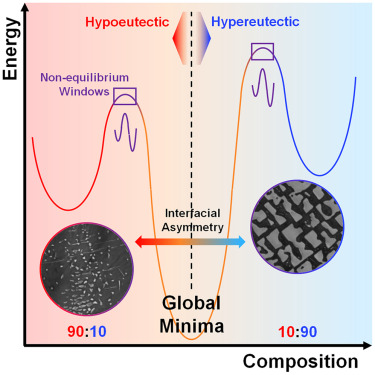
Their research demonstrates that non-eutectic metal alloys undergo in situ partitioning, speciation, and segregation when moved away from equilibrium. These processes create new interfaces that introduce interfacial stress, generating metastable microstates and delaying conventional phase transitions. The result is not simply a phase change—but the emergence of complexity from disorder, governed by enthalpy and entropy divergence.
The team analyzed the evolution of heat capacity, enthalpy, and entropy in these systems and observed asymmetric enthalpy dissipation during phase transitions. This asymmetry reinforces the idea that internal structuring—driven by non-equilibrium conditions—alters the thermodynamic pathway, suppressing crystallization and prolonging the liquidous state.